Keywords: Kidney, Nephrostomy, Percutaneous, Ultrasound, Fluoroscopy
ABSTRACT
Objectives: Percutaneous nephrostomy (PCN), an indispensable adjunct in the treatment of obstructive uropathy of various etiology, has been traditionally performed under fluoroscopic guidance. This study aims to ascertain the effectiveness and safety of ultrasound-guided PCN in actual patients by determining the success and procedure-related complication rates and comparing them with fluoroscopic-guided procedure.
Materials and Methods: 65 formal and large-bore ultrasound-guided PCN was performed on 50 consecutive patients. A non-concurrent group of 62 patients previously underwent 68 procedures under fluoroscopy. Uniform instrumentation and technique consisting of initial needle access, guidewire insertion, tract dilatation, and placement of nephrostomy tube (Foley catheter) with size ranging from French 12 to 16 were utilized. The incidence of successful tube placement and complications were observed in each group and compared using Chi-square statistic with Yates’ correction. Statistical tests were two-tailed and the level of significance was placed at p = 0.05.
Results: The overall success rate was 54 out of 65 (83.1%) and 63 out of 68 (92.6%) in the ultrasound and fluoroscopy groups, respectively (p=0.0899). Complications occurred in 7 patients (10.8%) in the ultrasound group and 6 patients (8.8%) in the fluoroscopy group (p=0.7056). The most common complication was significant bleeding: 4 (6.2%) in the ultrasound group and 3 (4.4%) in the fluoroscopy group. Periorgan injury was observed in 2 (2.9%) fluoroscopy and 1 (1.5%) ultrasound patient.
Conclusions : The type of imaging modality employed in PCN does not appear to create an impact in the overall success and procedure-related complication rates. Formal PCN utilizing a Foley catheter as large as French 16 may then be effectively and safely performed solely under ultrasound guidance without any significant failure or increase in morbidity compared to a fluoroscopy-guided procedure. This technique is particularly useful in settings where fluoroscopy is inaccessible or when exposure to radiation is deemed perilous to the patient.
Introduction
Renal surgery has not been spared from the recent technological advancements which paved way to the acceptance of non or minimally-invasive procedures. Access to the kidney such as percutaneous nephrostomy (PCN) has largely replaced the decreasing and less popular open technique. Introduced by Goodwin in 1955 and refined until it became a routine practice in the 1980’s, PCN is now considered an essential component in the treatment of upper urinary tract obstruction when a retrograde route proves anatomically or technically unattainable.1 Likewise, it is the established initial step in obtaining antegrade access to the kidney for a melange of procedures including nephroscopy, percutaneous nephrolithotomy. antegrade endopyelotomy and many others.2 It is also widely utilized as a permanent urinary drainage for patients with decompensated lower urinary tract particularly those with malignant obstruction of the ureter or urinary bladder or advanced genitourinary tuberculosis.3 These divergent applications have made PCN a prevalent and indispensable urologic procedure being performed nowadays.
The standard technique fundamentally involves an initial renal puncture, placement of a guidewire, tract dilatation, and the insertion of a nephrostomy tube with the desired caliber. The success and safety of the procedure depends on several factors, namely: the operator and his technical acumen, the patient himself – anatomic variations and degree of hydronephrosis, and proper instrumentation.4 Of cardinal importance is optimal visualization of the urinary tract particularly during the initial placement of a needle access. Inasmuch as the development of PCN was conceived employing fluoroscopy, this remains to be the recognized standard imaging modality.2 The role of real-time ultrasonography has been limited to initial needle puncture, during emergency small-bore nephrostomy tube placement for acute obstruction and sepsis, for severely hydronephrotic kidneys, or for procedures wherein radiation exposure is contraindicated such as pregnancy.5,6,7 However, the development of high resolution ultrasound models and its increasing familiarity among trained ultrasonologists have created a profound impact on urologic imaging over these recent years.4 Its application to the evaluation of the kidney has been refined such that it can allow precise determination of kidney size, cortical thickness and echogenecity, corticomedullary differentiation and assessment of the pelvocalyceal system for the presence of hydronephrosis. In contrast to fluoroscopy, it has the main advantage of being easy to perform, causes little or no patient discomfort, does not use ionizing radiation or contrast media, relatively inexpensive and ubiquitously available. As a guide during PCN, ultrasound has a continuous real-time control of puncture; is able to image radiolucent, non contrast-enhancing renal and extrarenal structures for puncture; can image tissues along an intended nephrostomy tract (for example, bowel and lung), can image in numerous planes by simply shifting, tilting and rotating the scanning head and lastly, it offers a three-dimensional information during puncture: if the two-dimensional image plane shows both the target and the puncture needle (which has to be directed and aligned accordingly), the position of the needle remains confined to the volume of the slice of the scan without lateral deviation of the needle into the third dimension.4
With these refinements and technical cognizance on ultrasonography, its application to percutaneous renal procedures may not only be limited to small-caliber PCN or to initial needle puncture during formal PCN, but also be of benefit during tract dilatation and placement of a large bore nephrostomy tube, with distinct advantages over that of fluoroscopy.
Thus, this paper aims to determine the effectiveness and safety of ultrasound as an alternative imaging technique during formal PCN by measuring its success and complication rates in actual group of patients and comparing these with a non-concurrent but clinically similar fluoroscopy group.
Materials and Methods
50 patients with obstructive uropathy secondary to urolithiasis and benign or malignant obstruction of the lower urinary tract underwent formal PCN under ultrasound guidance. All patients had normal or corrected bleeding parameters such as prothrombin, partial thromboplastin, clotting and bleeding times, including platelet count; had sterile urine or received a broad spectrum antibiotic for at least 24 hours before the procedure; and had hemoglobin levels of at least 10 mg/dl, or when less, have been transfused with the appropriate blood component. These were standard requirements in the institution for every patient undergoing PCN regardless of the type of imaging modality employed. Bilateral procedures were performed on 15 patients, one of whom under local anesthesia. All the rest were operated under general anesthesia. One patient underwent subsequent percutaneous nephrolithotomy solely under ultrasound guidance. To minimize further patient variability, procedures performed on emergency basis, PCN’s wherein small-bore nephrostomy tubes (French 8 or less) were used, or single-step PCN’s without tract dilatation were not included in the study.
The entire procedure was a cooperative effort between a urologist who undertook the actual renal access and a radiologist who manipulated the ultrasound probe. Inasmuch both private and service patients comprised the study groups, and that the training program aimed to equally distribute PCN experience among residents, the operator was not limited to a single surgeon. Hence, operator variablity was also compared. Patients were placed in a prone position with slight elevation of the lumbar area and flexion of the legs. The initial puncture was approached through the most accessible posterior calyx with the shortest distance from the skin, following the landmarks and the technique described by Thuroff.4 The actual direction and depth of the needle were determined ultrasonographically using a SiemensTM portable ultrasound machine with a 3.5 MHz transducer. Puncture was made using a 16-gauge Chiba needle followed by introduction of a floppy tip or Amplatz 0.038-inch guidewire with its tip directed towards the renal pelvis or proximal ureter. For patients with mild hydronephrosis, a two-stick technique was employed: A 20 or 22-gauge spinal needle was placed medially and perpendicularly along the coronal plane to access the kidney, its route and depth also determined by ultrasound, then a sterile normal saline was infused through the needle to cause a certain degree of hydronephrosis adequate for a clear placement of the Chiba needle in the pelvis or desired calyx. Tract dilatation was done using sequential, semi-rigid Amplatz or telescoping metal dilators. The tract was dilated up to 2 or 4 French- size larger than the catheter to be placed. Catheters were of the Foley type with size ranging from 12 to 16 French. Post-PCN antegrade nephrostogram was done in all patients to check catheter position and for other diagnostic purposes.
The fluoroscopy group involved 62 patients who previously underwent 68 procedures in our institute from July, 1994 to June, 1996. A PhilipsTM Fluoroscope with C-arm was utilized. The approach was essentially similar to that of the ultrasound group. 25 patients had an initial retrograde ureteral catheter placement and injection of iodinated contrast medium through the catheter inorder to opacify or distend the collecting system. 22 of the patients underwent subsequent percutaneous nephrolithotomy or nephrolithotripsy. Any event arising from stone manipulation or extraction were not included in the comparison since these were not performed on the ultrasound group.
The procedure was considered successful when antegrade nephrostogram would show the catheter tip inside the preferred position in the collecting system, in addition to an egress of urine from the catheter. Failure was recorded when catheter placement was unattainable due to any of the following reasons: the collecting system could not be accessed through the initial puncture; the correct tract was "lost" during dilatation; the catheter could not be properly positioned even after tract dilatation; the procedure had to be terminated due to complications; or the catheter tip was not within the renal collecting system as demonstrated by antegrade nephrostogram. Procedure-related complications occurring with successful or unsuccessful tube placement were monitored and these include: acute hemorrhage requiring transfusion or open control, periorgan injury, septicemia, or complications related to technique and instrumentation such as guidewire disruption requiring open retrieval. Minor complications like clogging of the catheter, catheter displacement secondary to patient activity, deflation of the catheter balloon, or other long-term complications were not included in the assessment.
The incidence or proportion of successful catheter placement and complications were noted and compared between each group using Chi-square statistic with Yates’ correction. All tests of hypotheses were two-tailed and p values lower than 0.05 were considered statistically significant. A CDC/Epi Info Software was utilized in the calculations.
Results
Overall, a total of 112 patients with 133 procedures were studied with a male to female ratio of 1:1.6. The mean patient age was 45.5 years (range, 16 –76). Senior urology residents performed 64% of the procedures, the remaining, by consultants. A bilateral procedure was done on 21 of the patients. The etiology of obstructive uropathy was stone disease in 64 patients, 52% of whom had staghorn stones. 35 patients had malignant obstruction of the urinary tract, 53% of whom were due to cervical carcinoma. 6 patients had genitourinary tuberculosis with severe involvement of the ureter or urinary bladder. A comparison of these demographic and clinical characteristics of patients between the ultrasound and fluoroscopy groups is summarized in Table I. There was no statistically significant difference in terms of age (p=0.0569), sex (p=0.938), laterality of the procedure (p=0.8162) or consultant to resident ratio (p=0.359). In the ultrasound group, however, the clinical diagnosis comprised of malignancy in 50% (cervix, colon, urinary bladder, prostate - in descending order) whereas in the fluoroscopy group, 74% was due to stone disease and 16% was due to malignancy (p=0.0001).
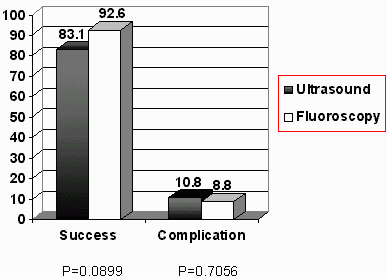
The combined success rate was 117 out of 133 (88.0%) and overall complication rate was 13 out of 133 (9.8%). Comparison of these variables between each group is shown in Table II and Figure 11. Among the ultrasound group, tube placement was successful in 54 out of 65 procedures (83.1%), 4 of whom developed complications. In the fluoroscopy group, 63 out of 68 procedures (92.6%) were successful, 2 of whom developed complications. Complications were also observed even when the nephrostomy tube was not successfully placed. This was seen in 3 patients in the ultrasound group and 4 in the fluoroscopy group. A list of the patients who developed complications and the corresponding management is detailed in Table III. The most common complication was acute bleeding which required blood transfusion or open control of hemorrhage. This was noted in 7 patients (5.3%), 4 and 3 in the ultrasound and fluoroscopy groups, respectively. Periorgan injury was noted in 3 patients (2.3%), 1 in the fluoroscopy group subsequently expired after a week in the intensive care unit. One patient in the ultrasound group also expired due to sepsis thus setting a procedure-related mortality rate of 1.5%.
A comparison of the proportion of success and complication between the two groups was performed. Statistical test of the difference is shown in Tables IV and V. The p value obtained in each table was more than 0.05 indicating no statistically significant difference in the proportion of successful PCN or complications between the ultrasound and fluoroscopy groups.
Discussion
Percutaneous nephrostomy is now an established stock in the urologic armamentarium with a success rate of at least 90%2. This figure however, is based mainly on fluoroscopic-guided procedures performed by interventional radiologists in their own fluoroscopy units without the participation of a urologist. The 92.6% success rate obtained in our fluoroscopy group approximates this estimated figure with one major difference: a urologist performed the actual puncture and catheter placement. This contrasts to the western practice wherein radiologists play an active and independent role in percutaneous renal access.
Ultrasound-guided PCN, on the other hand, has been estimated to have lower success rate.4 The result in our ultrasound group (83.1%) simulates this observation. This may appear lower than the rate obtained by fluoroscopy although no statistical difference is actually observed. In our study, the procedure was a cooperative effort of both urologist and radiologist, whereas just like fluoroscopic-guided PCN in western practice, each specialist usually performs the procedure on his own, with the radiologist performing most of the operation. This apparent paucity of ultrasound-guided PCN being performed by urologists is further brought into fore by a retrospective review on 100 patients done by Mahaffey, et.al. in 1994 which was commented by Smith to be the first large series on PCN ever performed solely by urologists.1,9 Notwithstanding, as pointed out previously, technical acumen is not the sole determinant of an effective PCN. Renal anatomical variations, the degree of hydronephrosis and proper instrumentation all influence results. The success rates obtained in this study demonstrate that PCN appears reasonably effective regardless of the type of imaging modality involved.
Procedure-related complications continue to be widely reported regardless of the type of imaging employed.1-5 Rates vary from 25 – 60% though this includes late (more than 24 hours after insertion) and minor complications such as those related to tube malfunction, leakage, dislodgment and incrustations.1 Based on the 1986 work by Picus, Clayman, et.al. summarized early complications inherent with fluoroscopy-guided PCN, the most common of which are: acute bleeding requiring transfusion (<5%), failed access (<5%) , periorgan injury : bowel, spleen, lung (<1%), and septicemia (<1)2. On the other hand, Mahaffey, et. al., listed down the most common early complications of ultrasound-guided PCN as sepsis (3%), renal pelvic perforations (2%) and hemorrhage (1%).1 Comparing these figures with those obtained by radiologists utilizing fluoroscopy, Mahaffey concluded that percutaneous nephrostomy tubes may be placed safely by urologists under ultrasound guidance. This conclusion deviates from an earlier report by Zegel, et.al., in 1981 emphasizing the need to perform percutaneous procedures under fluoroscopy.8
Despite the bias in this study’s historical fluoroscopy group, our 8.8% complication rate does not digress from the aforementioned retrospective reports, albeit the 10.8% complication rate obtained in our ultrasound group may appear higher. Overall, significant hemorrhage requiring transfusion or surgical control ranks first among the complications (5.3%) and is equally distributed among both groups. This event can be attributed to a relatively thick renal parenchyma which can be injured during tract dilatation particularly when excessive amount of torque and instrument angulation are applied.9 According to Davidoff and Bellman, instrumentation, largely influences the occurrence of such event which may be minimized by utilizing balloon dilatation systems instead of the traditional sequential Amplatz dilators.10 The type of imaging modality employed does not appear to have a downright bearing on the incidence of hemorrhage, inasmuch as neither fluoroscopy nor ultrasound offers direct visualization of renal vascularity.
Moreover, our results show that significant bleeding occurred in patients with associated stones (3 and 2 in the ultrasound and fluoroscopy groups respectively). While this is usually manageable by means of transfusions, open control remains an option and in such instance open stone surgery may then be performed thereby recalling the need for other forms of stone therapy such as extracorporeal shock wave lithotripsy. Minor bleeding is common but usually resolves spontaneously with observation and occasional flushing of the catheter.
Periorgan injury ranks second among the complications and thus poses a similar concern. In the fluoroscopy group, the colon and pleura were inadvertently injured. The former necessitated a colostomy, the latter, a tube thoracostomy. Pleural tear has been deemed more common in instances wherein the nephrostomy tract is placed more superiorly, particularly when subsequent stone manipulation is contemplated.2 Duodenal perforation occurred in one ultrasound patient but this was managed conservatively with parenteral nutrition and readjustment of the nephrostomy tube under fluoroscopy. Similarly, the type of imaging modality does not appear to make any impact in the prevention of these injuries. A thorough knowledge on the anatomical relationships and variations and topographical landmarks remain a more effective preventive key for such events.11 Gentle instrument manipulation can not be overemphasized.
Septicemia, the underlying cause of death in two of our patients, occurred in both ultrasound and fluoroscopy groups in spite of urinary sterilization. The coexistence of co-morbidities such as perirenal abscess or bowel perforation may have contributed chiefly to this occurrence. Prevention of overinflation of the collecting system which can force organisms into the renal tissues and vessels or gentle manipulation of the dilators with particular care not to exceed the depth of puncture are two important reminders related to the need of an adequate technical prudence on the part of the operator.9
These aforementioned complications being present equally in both ultrasound and fluoroscopy-guided PCN may simply indicate no distinction between the two in as far as patient safety is concerned. Overall patient status, skill, technique and instrumentation would probably play a more significant role.
Most urologists’ preference on fluoroscopy over ultrasound has been based on the fact that the latter does not offer a direct visualization of the organs of interest, but rather, depend on the sound waves emitted by them.5 Thus, inter-operator variability or the so-called "operator dependence" of ultrasound has prevailed over its advantages. One important advantage observed in this study is the capacity of ultrasound to allow a more precise placement of the needle on the intended plane of access without retrograde opacification of the collecting system or resorting to blind needle access. As long as the needle and collecting system are visible on the monitor, i.e., both are seen on a single "slice", it can be ascertained that the needle is on the right plane. In contrast to the two-dimensional view in fluoroscopy, although both needle and collecting system may be visible on the monitor, this does not rule out the possibility of ventral or dorsal needle displacement. Thus, it has been the common practice to utilize ultrasound in the initial needle access even during fluoroscopic-guided PCN.11
Paraphrasing Amplatz, a preeminent interventional radiologist, Smith claims that "ultrasound is similar to artificial insemination; it is very good but not the real thing,"9 Disregarding the obvious disadvantages of fluoroscopy, namely radiation exposure and added expense, it is of no debate that fluoroscopy, in addition to ultrasound would have better results, all other factors being equal. The issue becomes immaterial, however, when inaccessibility to the "real thing" poses management delay and compromises patient care. In such instance, the decision to perform PCN under ultrasound guidance may no longer be a matter of choice, but rather a matter necessity.
Conclusions
The type of imaging modality employed in formal, large-bore percutaneous tube nephrostomy does not appear to create any impact on the overall success and procedure-related complication rates. PCN utilizing a Foley catheter as large as French 16 may then be effectively and safely performed solely under ultrasound guidance without any significant failure or increase in morbidity compared to a fluoroscopy-guided procedure. This management offers a practical alternative in the antegrade treatment of obstructive uropathy particularly in areas where fluoroscopy is inaccessible or when radiation exposure is deemed unsafe or contraindicated.
List of Tables
Table I. Comparison of Demographic and Clinical Characteristics of Patients| ULTRASOUND | FLUOROSCOPY | P VALUE | |
| Total Patients | 50 |
62 |
-- |
| Total Procedures | 65 |
68 |
-- |
| Mean Age in Years (+/- SD) | 48 (+/-14.7) |
43 (+/-15.3) |
0.0569 |
| Sex – Male:Female Ratio | 19 : 31 |
24 : 38 |
0.9380 |
Diagnosis:
|
18 25 4 3 |
46 10 2 4 |
0.0001 |
Laterality Of Procedure
|
16 19 15 |
27 29 6 |
0.8162 |
Surgeon: Consultant To Resident Ratio |
26 : 39 |
22 : 46 |
0.3590 |
Variable |
Ultrasound (n=65) |
Fluoroscopy (n=68) |
Successful PCN
|
54 (83.1%) 50 (76.9&%) 4 (6.2%) |
63 (92.6%) 61 (89.7%) 2 (2.9%) |
| Unsuccessful PCN |
11 (16.9%) 8 (12.3%) 3 (4.6%) |
5 (7.3%) 1 (1.5%) 4 (5.9%) |
| Overall complications | 7 (10.8%) |
6 (8.8%) |
Table III. List of Patients With Complications and Their Management
Patient Age/Sex |
Diagnosis | Complication | Management | ||
Ultrasound Group |
|||||
1. B.R. 49 F |
Stone Disease |
Bleeding |
Transfusion |
||
2. K.D. 46 F |
Cervical Ca |
Bleeding |
Open control, Open NT* |
||
3. V.C. 61 F |
U. Bladder TCCA |
Disrupted Guidewire |
Open retrieval, Open NT |
||
4. A.L. 44 M |
CRI, Stone Disease |
Perirenal Abscess, Sepsis |
Open Drainage, Antibiotic (Mortality) |
||
5. D.A. 59M |
Colonic Ca |
Duodenal Perforation |
TPN, NT readjustment |
||
6. T.S. 43 M |
Staghorn Stone |
Bleeding |
Transfusion, Open NT |
||
7. L.B. 35 M |
CRI, Stone Disease |
Bleeding |
Transfusion |
||
Fluoroscopy Group |
|||||
1. A.D. 40 F |
Staghorn Stone |
Bleeding |
Open control, Pyelolithotomy | ||
2. J.A. 39 M |
Inf. Calyceal Stone |
Bleeding |
Open control, Nephrolithotomy | ||
3. S.E. 58 F |
Cervical Ca |
Bleeding |
Cysto, Clot Evacuation, Transfusion | ||
4. F.A. 73 M |
U. Bladder TCCA |
Renal Pelvic Perforation |
Open NT, Pelvorrhaphy |
||
5. T.G. 60 M |
Inf. Calyceal Stone |
Pleural Perforation |
Open NT, Tube Thoracostomy | ||
6. L. R 43 F |
Pelvolithiasis |
Bowel Perforation Ac. Pancreatitis, Sepsis |
Colostomy, Antibiotic (Mortality) | ||
*Nephrostomy Tube
Table IV. Computation of the Chi-square Statistic For Proportion of Success
Group |
Success | No. Observed (o) | No. Expected (e) | o – e | Contribution to Chi square (o-e)2/e |
Ultrasound |
Yes |
54 |
57.2 |
-3.2 |
0.018 |
No |
11 |
7.8 |
3.2 |
1.313 |
|
Fluoroscopy |
Yes |
63 |
59.8 |
3.2 |
0.171 |
No |
5 |
8.2 |
-3.2 |
1.249 |
|
Total |
133 |
133.0 |
2.751 |
Degree of freedom (df) = 1
2.751 < 3.84 at 1 df
p = 0.0899
Table V. Computation of the Chi-square Statistic for Proportion of Complications
Group |
Complication |
No. Observed (o) |
No. Expected (e) |
o – e |
Contribution to Chi square (o-e)2/e |
Ultrasound |
Yes |
7 |
6.4 |
0.6 |
0.06 |
No |
58 |
58.6 |
0.6 |
0.01 |
|
Fluoroscopy |
Yes |
6 |
6.6 |
-0.6 |
0.05 |
No |
62 |
61.4 |
-0.6 |
0.01 |
|
Total |
133 |
133.0 |
0.13 |
Degree of freedom (df) = 1
0.13 < 3.84 at 1 df
p = 0.7056
Figure 11. Comparison of Overall Success and Complication Rates Between Ultrasound and Fluoroscopy Groups

ANDRES S. MARRERO, M.D. JOSE BENJAMIN L. MENDOZA, M.D.
Division of Urology, National Kidney and Transplant Institute, East Avenue, Quezon City, Philippines 1100
Copyright 1999 National Kidney and Transplant
Division of Urology, Philippines
All Rights Reserved.